
Since H is the only remaining unbalanced element, in the next step, we will put a 2 in front of H2O to try to balance it. To balance the given equation, put a 3 in front of the HNO 3 on the left hand side to get 3 N's on both sides:Īs a side-effect, O was also balanced with 9 on each side. This equation is not balanced because there are an unequal amount of H's, N's and O's on both sides of the equation. However, the left hand side has 1 H, 1 N, and 3 O's and the right hand side has 2 H's, 3 N's and 9 O's. The left hand side has 1 Cu and the right hand side has 1 Cu, so the Cu atoms are balanced. Now that we've hopefully gotten the hang of balancing simple chemical equations, lets move onto something a bit more complex, such as the reaction of Copper (Cu) and Nitric Acid (HNO3) into Copper(II) Nitrate (Cu(NO3)2) + Nitrogen Dioxide (NO2) + Water (H2O):
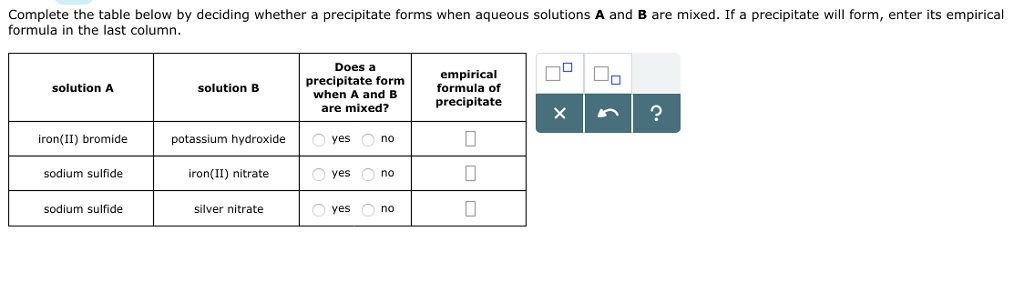
#Solution precipitate calculator trial
We could have tried, but we would have realized that we would need to balance H again (hence the trial and error). You might wonder why we didn't instead increase the number of H2 molecules. As a next step, lets balance H again by putting a 2 in front of NaOH so the equation reads:
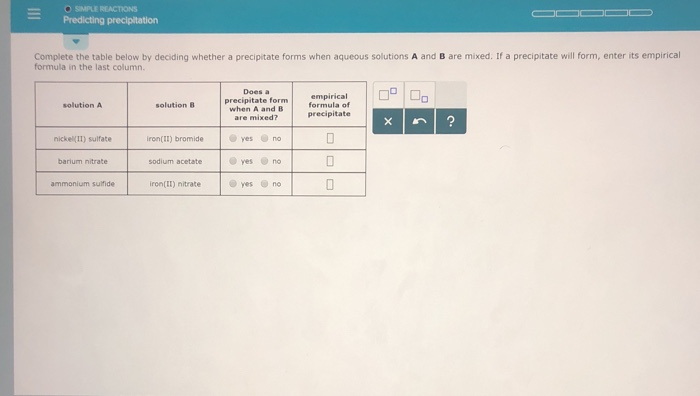
#Solution precipitate calculator update
This is common and doesn't mean any mistakes were made.Īs was mentioned before, we knew we had to update multiple coefficients to balance the hydrogen atoms. We went from 1 unbalanced element to multiple. We now have 4 H, 2 O and 1 Na atom on the left, but 3 H, 1 O and 1 Na atom on the right. Notice how the 2 in front of H2O is "distributed" to both the H 2 and the O. Because 3 is not divisible by 2, it means coefficients of multiple compounds containing H will need to be changed. In this case, there are an equal number of Na and O atoms, but like last time, H needs to be balanced, with 2 on the left, and 3 on the right. Take for example the exothermic reaction of Sodium (Na) and Water (H2O), which releases heat, Sodium Hydroxide (NaOH) and Hydrogen Gas (H2): Usually, balancing chemical equations will require multiple steps.
The previous example was simpler than most but demonstrated the basic concepts. That is the case in chemical equations like: Zinc + Hydrogen Chloride = Zinc Chloride + Hydrogen Gas: Zn + HCl → ZnCl2 + H2Īlso, be aware that sometimes no balancing is needed.Iron + Hydrogen Chloride = Ferrous Chloride + Hydrogen Gas: Fe + HCl → FeCl2 + H2.Now that there is an equal quantity of Ca, C, Cl, H and O on both sides, the chemical equation is balanced.Įven though it was simple, there are actually quite a few cases where you can balance the chemical equation in one step: To balance H and Cl, we can put a 2 in front of HCl on the left-hand-side: There is 1 H and 1 Cl on the left, but 2 of each the right. If we count up the number of each element on both the left-hand-side and right-hand-side, we see that all but Hydrogen and Chlorine are balanced. To start off with a simple example, lets balance the acid-base reaction of two salts, Calcium Carbonate (CaCO3) + Hydrochloric Acid (HCl) → Calcium Chloride (CaCl2) + Carbon Dioxide (CO2) + Water (H2O): The basic idea is to balance one element at a time (usually starting with the most complicated molecule, and ending with hydrogen and oxygen) until all the elements are balanced. The easiest way for beginners to balance simple chemical equations by hand is via inspection (also called trial and error). Balance Using Inspection + Linear Systems.


 0 kommentar(er)
0 kommentar(er)
